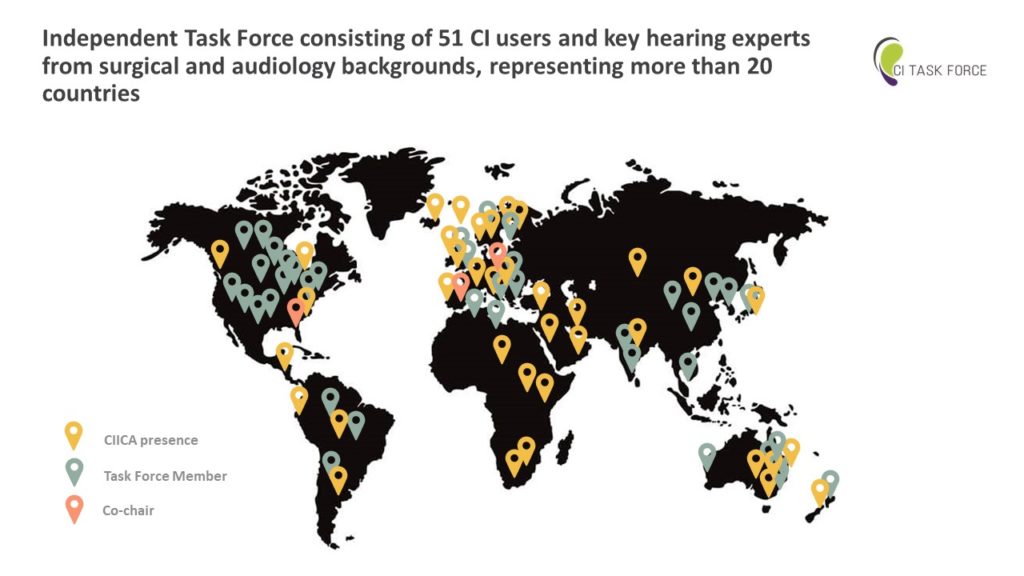
The 2023 Standards of Care for hearing health includes all of the current clinical practice recommendations and is intended to provide clinicians, patients, researchers, payers, and others with the components of hearing care, general treatment goals, and tools to evaluate the quality of care. The recommendations are based on an extensive review of the clinical hearing literature, supplemented with input from the CI Task Force and Hearing Health Collaborative members and the medical community at large.
The Standards of Care for hearing health are updated annually, or more frequently online if new evidence changes merit immediate incorporation.